Answer:
Option A,B,D
Explanation:
Statement wise explanation is
Statement (a)
[Co(en)(NH3)3H2O]3+ has the following 2 geometrical isomers.
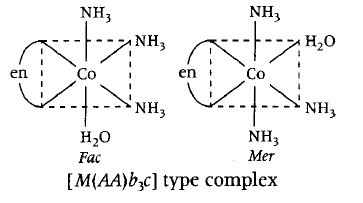
Hence, this is the correct statement.
Statement (b)
If bidentate ligand 'en' is replaced by two cyanide ligands then
[Co (NH3)3(H2O) (CN)2]+ is formed.
It is [Ma3b2c] type complex which has the following 3 geometrical isomers.
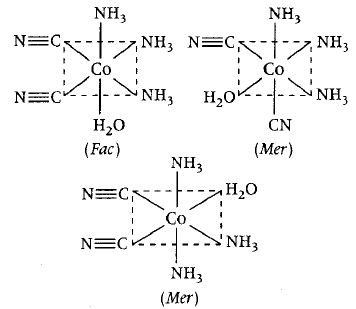
Hence, this statement is also correct.
Statement (c)
Co metal has [Ar] 3d74s2 configuration while in [Co(en) (NH3)3 (H2O)]3+ , It is in +3 oxidation state . Thus, Co3+ has [Ar]3 d6 configuration, and d2 sp3 in complex.
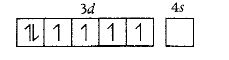
As en is a strong ligand, so pairing will occur

Due to the presence of unpaired electrons it shows diamagnetic behavior rather than paramagnetic.
Hence, the statement is incorrect.
Statement (d)
According to CFT, absorption of light by coordination complexes depends upon CFSE i.e, crystal field splitting energy (Δ0) as
$\triangle_{0}\alpha\frac{1}{\lambda}$
Among the complexes given [Co(en) (NH3)4]3+ has more Δ0 value as compared to complex [Co(en) (NH3)3 (H2O)]3+ . Thus , [Co (en) (NH3)3(H2O)]3+ absorbs the light at longer wavelength for d-d transition. Hence , this statement is also correct.
Note. For any complex , the value of Δ0 can be calculated via the difference or gap between eg and t2g values.